Atomic structure refers to the organization and arrangement of subatomic particles within an atom. Atoms are the basic building blocks of matter. These are composed of three main subatomic particles: protons, neutrons, and electrons.
Table of Contents
An overview of Atomic structure
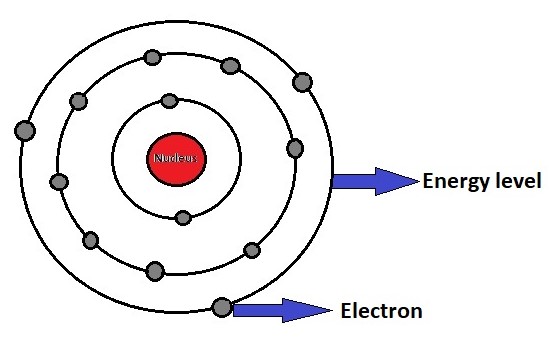
Nucleus:
At the center of an atom is the nucleus, which is tiny but extremely dense. The nucleus contains two types of subatomic particles:
1. Protons (p)
Protons are positively charged particles found in the nucleus (center) of an atom. Each proton has a charge of +1 and a mass of approximately 1 atomic mass unit (amu). The number of protons in the nucleus defines the element and gives it its unique atomic number.
2. Neutrons (n)
Neutrons are electrically neutral particles also located in the nucleus of an atom. They have a mass similar to protons, approximately 1 amu, but they carry no electric charge. The number of neutrons in an atom can vary for a given element, resulting in different isotopes of that element. The formula to find out the number of neutrons is given by below formula.
Number of Neutrons = Atomic mass – Number of Protons
Electrons (e–)
Surrounding the nucleus is a cloud of electrons. Electrons are negatively charged particles that move in specific energy levels or electron shells around the nucleus. They have a much smaller mass compared to protons and neutrons, about 1/1836 amu. Electrons are responsible for chemical reactions and the interactions between atoms. The arrangement of electrons in these energy levels is crucial in determining an element’s chemical properties.
The organization of electrons in electron shells is described by various models, such as the Bohr model and the quantum mechanical model. In the quantum mechanical model, electrons are described as existing in electron clouds or orbitals, which represent regions of probability where an electron is likely to be found. These orbitals are grouped into energy levels or shells, with each shell accommodating a specific number of electrons.
Each electron shell can hold a specific number of electrons. These electron shells, or energy levels, are denoted by quantum numbers (n = 1, 2, 3, etc.). Electrons occupy the innermost shell first before moving to higher energy shells. This distribution follows the Aufbau principle, Pauli exclusion principle, and Hund’s rule.
Rules for distribution of electrons
The distribution of electrons in the electron shells (energy levels) of an atom follows several rules, which are based on the principles of quantum mechanics. These rules help us understand how electrons are arranged within an atom:
1. Aufbau Principle:
Aufbau Principle states that electrons fill the lowest energy orbitals (electron shells) first before moving to higher energy ones. In other words, electrons occupy the innermost shells before filling the outermost shells that is electrons are filled in order of increasing energy of orbitals (n+l). This is similar to how we fill seats in a theatre from the front row to the back row.
Example 1: In which orbital the electron will enter, 1s OR 2s ?
1s = (n+l) = 1+0 = 1 & 2s = (n+l) = 2+0 = 2
Since 2 is greater than 1 & therefore energy of 2s orbital is more and the electron will enter first in 1s and then only in 2s orbital.
Example 2: In which orbital the electron will enter, 4f OR 5d ?
4f = (n+l) = 4+3 = 7 & 5d = (n+l) = 5+2 = 7,
Orbital of lower principal quantum number is considered of lower energy & therefore electron will enter in 4f orbital.
2. Pauli Exclusion Principle:
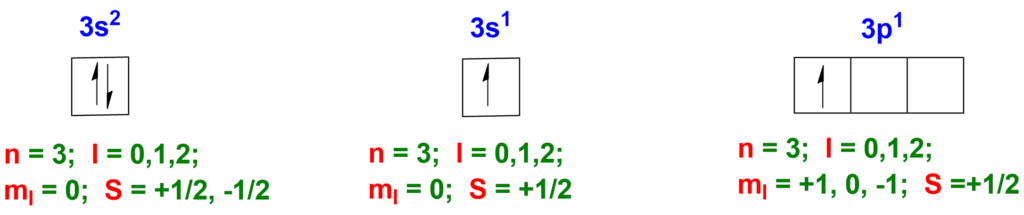
The Pauli Exclusion Principle states that no two electrons in an atom can have the same set of four quantum numbers. These quantum numbers include the principal quantum number (n), the azimuthal quantum number (l), the magnetic quantum number (ml), and the spin quantum number (ms). In simple terms, it means that within a given energy level and sublevel, each electron must have a unique combination of quantum numbers, and no two electrons can have the exact same quantum numbers.
Example: 3s2 , 3s1 , 3p1
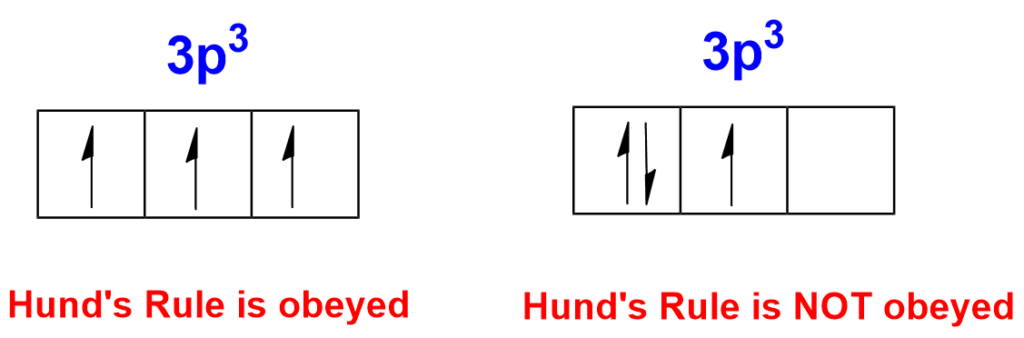
3. Hund’s Rule:
Hund’s Rule dictates that electrons occupy degenerate (equal-energy) orbitals in a sublevel singly before pairing up. In other words, when there are multiple orbitals with the same energy (e.g., p orbitals), electrons will first enter these orbitals with parallel spins (i.e., having their “up” and “down” spin) before pairing up. This minimizes electron-electron repulsion and leads to greater stability.
4. Maximum Number of Electrons in Each Shell:
The maximum number of electrons that can occupy a given shell is determined by the formula 2n2, where ‘n’ is the principal quantum number of the shell.
For example:
- The first shell (n = 1) can hold a maximum of 2 electrons.
- The second shell (n = 2) can hold a maximum of 8 electrons.
- The third shell (n = 3) can also hold a maximum of 8 electrons, and so on.
5. Maximum Number of Electrons in Each Sublevel:
Within a given shell, there are sublevels or subshells, which are designated by letters (s, p, d, f, etc.). Each sublevel has a different number of orbitals and can hold a specific number of electrons:
- The s sublevel can hold a maximum of 2 electrons.
- The p sublevel can hold a maximum of 6 electrons (in three orbitals).
- The d sublevel can hold a maximum of 10 electrons (in five orbitals).
- The f sublevel can hold a maximum of 14 electrons (in seven orbitals).
These rules govern the arrangement of electrons within an atom, leading to the unique electron configurations for each element on the periodic table. Electron configurations provide valuable information about an element’s chemical behavior and its location in the periodic table.
Key points about atomic structure
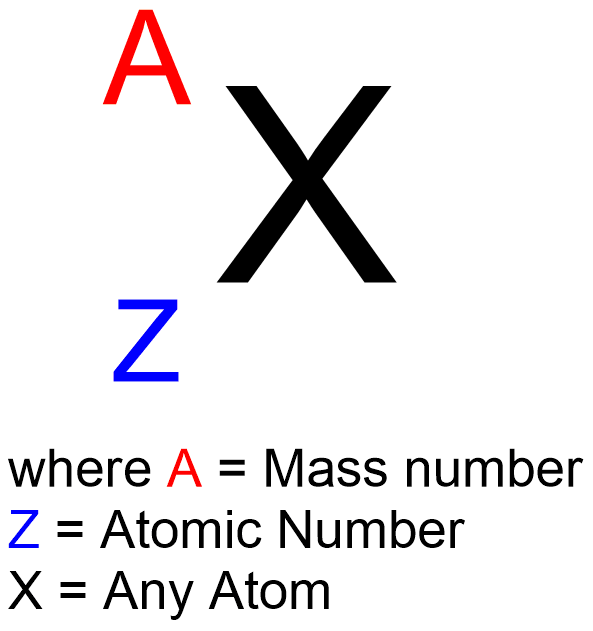
The atomic number of an element is equal to the number of protons in its nucleus and determines the element’s identity.
The mass number of an atom is the sum of its protons and neutrons.
Isotopes of an element have the same number of protons (and, therefore, the same atomic number) but different numbers of neutrons, resulting in different mass numbers. Examples of isotopes of hydrogen are protium, deuterium and tritium.

Examples of isotopes of chlorine are

Electrons in the outermost energy level (valence electrons) play a significant role in an element’s chemical reactivity and bonding with other atoms.
Atoms strive for stability by achieving a full outermost electron shell, typically through chemical bonding.
Learn more about inorganic chemistry in our category inorganic.


One comment
Comments are closed.