Chirality and optical activity are closely connected concepts in chemistry, particularly in the field of stereochemistry. It is an essential concept in many areas of chemistry, mainly in organic chemistry and biochemistry. Let’s explore how both are related to each other:
Table of Contents
Chirality
It is a concept in chemistry that describes the three-dimensional spatial arrangement of atoms within a molecule and its mirror image. It also explains about the molecules which are non-superimposable on their mirror images, much like our left and right hands and such molecules are called as Chiral molecules. The term “chirality” comes from the Greek word “cheir,” meaning hand.
Some of the key points include:
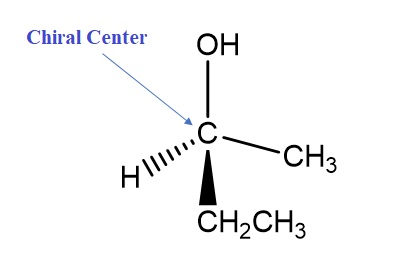
Chiral Centers/Atoms: Chirality often arises from asymmetric carbon atoms, where four different substituents are attached to a carbon atom, leading to non-superimposable mirror images.
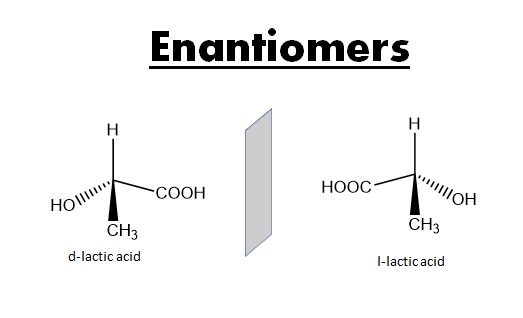
Enantiomers: Enantiomers are pairs of molecules that are mirror images of each other but not superimposable. They have opposite optical activities and rotate plane-polarized light in opposite directions. Enantiomers have the same physical and chemical properties, except when it comes to their interaction with other chiral entities, such as other chiral molecules or polarized light.
Racemization: A racemate or racemic mixture is a 1:1 mixture of enantiomers. Racemization refers to the process of interconverting an enantiomerically pure substance into a racemic mixture.
Chiral Compounds in Nature: Many biomolecules, such as amino acids and sugars, are chiral. The chiral molecules are crucial for their biological activity. For example, only one enantiomer of a chiral drug may be effective, while the other could be inactive or even harmful.
Chiral Catalysts: Chiral catalysts can selectively catalyze reactions to produce specific enantiomers of a product.
Achirality
It refers to the property of a molecule or an object that possesses a symmetry element, such as a mirror plane, an inversion center, or a rotation axis, allowing it to be superimposed onto its mirror image. In achiral entities, the mirror image is identical to the original, and the object lacks handedness.

This is a significant concept in chemistry, crystallography, and other scientific disciplines. Molecules with achiral characteristics often exhibit enhanced symmetry, making them predictable in terms of physical and chemical properties.
Evaluation of Chirality And Achirality
Symmetry elements play a crucial role in evaluating chirality in molecules. The presence or absence of certain symmetry elements provides information about the overall symmetry of a molecule and whether it is chiral or achiral.
Symmetry Elements like
- Mirror Plane (σ): If a molecule has a mirror plane (σ), it is achiral. A mirror plane means that one half of the molecule is a perfect reflection of the other half.
- Inversion Center (i): An inversion center results in an achiral molecule. The molecule is identical when rotated by 180 degrees around the inversion center.
- Rotation Axis (Cn): Rotation axes alone do not determine chirality. However, if a molecule lacks a mirror plane and has an odd-order rotation axis (Cn, where n is an odd number), it may be chiral. Even-order rotation axes do not leads to chiral.
A molecule is potentially chiral if it lacks a mirror plane, inversion center, or any other symmetry element. Symmetry elements that result in identical copies upon application (like a mirror plane or inversion center) lead to achirality.
- Chiral Molecules: If a molecule lacks all symmetry elements, it is potentially chiral. The absence of a plane of symmetry or an inversion center means that the molecule does not possess any symmetry that would make its mirror image superimposable.
- Achiral Molecules: If a molecule has a symmetry element, it is likely achiral. Symmetry elements allow for superimposability, making the molecule achiral.
Optical Activity
It is a property exhibited by certain substances (usually chiral compounds) that causes them to rotate the plane of polarized light passing through them. This phenomenon is a result of the interaction between the chiral molecules and the plane-polarized light. The degree and direction of rotation depend on the specific properties of the substance and the wavelength of light used.
Key points:
It is closely associated with chiral molecules. Chiral molecules, which lack a plane of symmetry, are capable of rotating the plane of polarized light. This property arises because the two enantiomers of a chiral molecule interact differently with circularly polarized light.
Enantiomers of a chiral compound exhibit opposite optical activities. One enantiomer will rotate plane-polarized light clockwise (dextrorotary or + rotation), while the other will rotate it counterclockwise (levorotary or − rotation). The specific rotation (α) is a measure of the amount and direction of optical rotation.
The specific rotation is a quantitative measure of optical activity. It is defined by the equation:
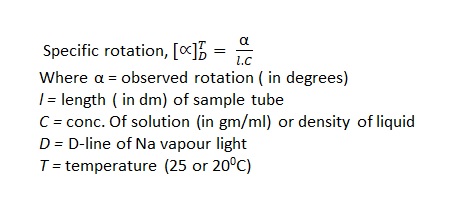
The extent of rotation (α) of the plane of polarization depends of
- Nature of the substance
- .Temperature
- Length ‘l’ through which light passes
- Density ‘d’ of the substance
- Wavelength of the light
The specific rotation is temperature-dependent, and it is usually reported at a specific temperature (commonly 20°C) using a standard concentration (usually 1 g/mL) and a specified wavelength of light.
Polarimeter
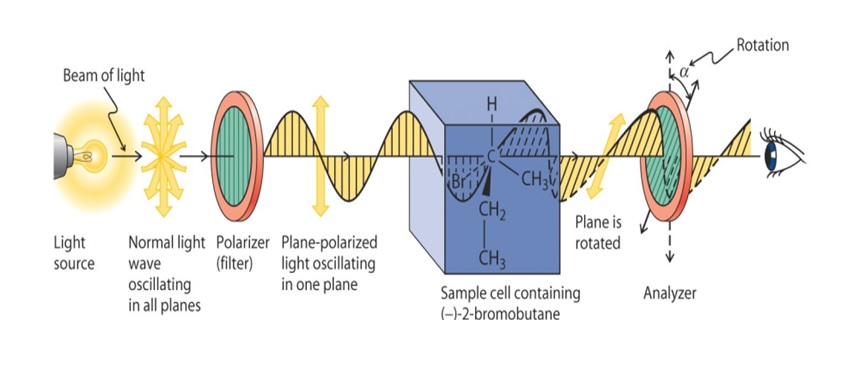
A polarimeter is the instrument used to measure optical activity. It typically consists of a light source, a polarizer to produce plane-polarized light, a sample tube where the substance is placed, and an analyzer to detect changes in the polarization of light after passing through the sample.
Applications in Chemistry and Pharmaceuticals
- Understanding the relationship between chirality and optical activity is crucial in various fields, including organic chemistry, biochemistry, and pharmaceuticals.
- In the pharmaceutical industry, it is often essential to produce and use specific enantiomers of a drug, as the biological activity or toxicity may be associated with a particular stereochemical configuration.
Summary
Chirality and optical activity are interconnected phenomena, and the study of optical activity provides valuable information about the three-dimensional arrangement of atoms in chiral molecules. This understanding is particularly relevant in fields where the biological effects of molecules depend on their stereochemistry, such as drug development and design. Do check more article on Bio In-organic in category.


One comment
Comments are closed.