Enantiomers and diastereomers are both types of stereoisomers, meaning they have the same molecular formula and connectivity of atoms but differ in their spatial arrangement. Separating both of these can be challenging due to their structural similarities. However, various techniques exist for the separation of these stereoisomers based on their unique properties and they exhibit distinct characteristics and differences:
Table of Contents
Enantiomers
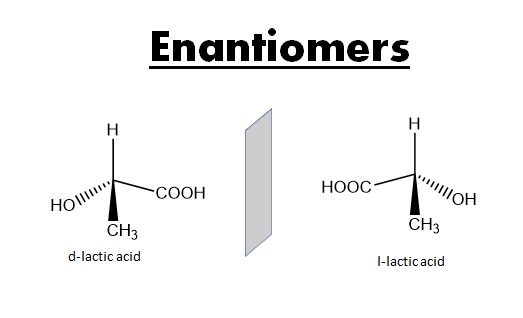
- Mirror Image Relationship:
Enantiomers are non-superimposable mirror images of each other. They have the same connectivity of atoms, but the arrangement of those atoms in three-dimensional space is like a left and right hand.
2. Chirality:

They are always chiral, meaning they lack an internal plane of symmetry. They typically involve chiral centers, and the molecules cannot be superimposed on their mirror images.
3. Number of Chiral Centers:
Arises from the presence of one or more chiral centers in a molecule. Chirality centers are carbon atoms bonded to four different substituents.
4. Optical Activity:
Exhibit equal but opposite optical activity, meaning one is dextrorotatory (rotates plane-polarized light clockwise), while the other is levorotatory (rotates plane-polarized light counterclockwise).
5. Relationship in a Mixture:
A mixture of enantiomers in equal proportions is called a racemic mixture. They interconvert through racemization. Racemization refers to the process by which an enantiomerically pure substance (containing only one enantiomer) becomes a racemic mixture.
6. Naming Conventions:
They are typically designated by the R/S nomenclature or the D/L system.
7. Biological Significance:
Can exhibit different biological activities. In biological systems, enzymes and other chiral molecules often interact selectively with one enantiomer over the other. This property is crucial in fields such as pharmacology, where the different effects of enantiomers need to be considered.
8. Identical Physical Properties:
Have nearly identical physical properties, including melting points, boiling points, and solubilities. This is because their overall chemical composition and connectivity are the same.
Separation Techniques Used:
- Chiral Chromatography: This technique utilizes a chiral stationary phase in the chromatographic column. Enantiomers interact differently with the chiral stationary phase, leading to differential retention times and separation.
- Enantiomer-Specific Reagents: Enantiomer-specific reagents, such as chiral derivatizing agents, can be used to convert the enantiomers into diastereomers which can then be separated using conventional methods.
- Chiral Additives: Adding chiral compounds to the mobile phase in chromatography can induce selective interactions with one enantiomer over the other, facilitating separation.
- Capillary Electrophoresis: Capillary electrophoresis exploits differences in the migration rates of enantiomers in an electric field based on their charge and size, allowing for separation.
- Enantioselective Crystallization: Some enantiomers can form diastereomeric crystals with chiral resolving agents, allowing for physical separation.
- High-Performance Liquid Chromatography (HPLC): Chiral stationary phases in HPLC columns can be employed for enantiomeric separation, similar to chiral chromatography.
- NMR Spectroscopy: Chiral solvating agents can be added to the sample for nuclear magnetic resonance (NMR) analysis, allowing differentiation between enantiomers.
Diastereomers
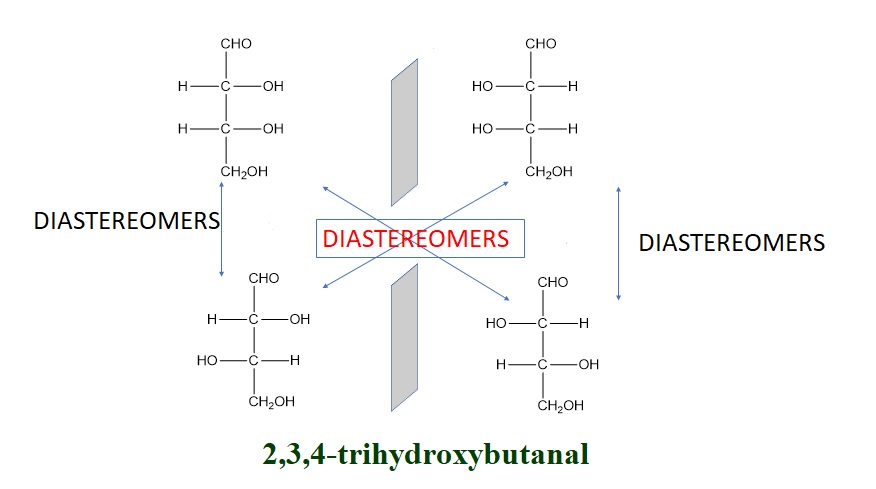
- Mirror Image Relationship:
Diastereomers are stereoisomers that are not mirror images of each other. They do not have the same arrangement of atoms in three-dimensional space but share some similarities.
2. Chirality:
They can be chiral or achiral. They may or may not have chiral centers, and even if they do, the molecules can have different spatial arrangements without being mirror images.
3. Number of Chiral Centers:
They may or may not involve chiral centers. They can arise from differences in the arrangement of atoms around one or more chiral centers, but they can also arise from other stereochemical differences.
4. Optical Activity:
Do not necessarily have equal and opposite optical activities. The optical activity of one diastereomer is not canceled out by the other.
5. Relationship in a Mixture:
A mixture of diastereomers is not necessarily in equal proportions, and they do not interconvert readily.
6. Naming Conventions:
They do not have specific naming conventions like R/S or D/L.
7. Biological Significance:
They can have different biological activities, and their distinct stereochemistry may lead to differences in how they interact with biological systems. This is particularly important in the field of drug development.
8. Different Physical Properties:
The physical properties like Melting Point, Boiling Point, density, etc of diastereomers can vary depending on their specific structural differences.
Separation Techniques Used:
- Chromatography: Having distinct chemical structures, often exhibit different affinities for a stationary phase in chromatography, leading to separation.
- Crystallization: Diastereomer may have different solubilities in certain solvents, allowing for separation through crystallization.
- Recrystallization: The process of recrystallization can be used to separate diastereomers based on their different solubilities during repeated dissolution and crystallization steps.
- Ion-Exchange Chromatography: Diastereomer with different charges can be separated using ion-exchange chromatography.
- Differential Reactivity: They may have different reactivities toward certain chemical reactions, allowing selective transformations for separation.
- Size Exclusion Chromatography: Differences in molecular size and shape can be utilized for separation using size exclusion chromatography.
- Selective Precipitation: Adding a selective reagent to a solution containing diastereomer can lead to the formation of a precipitate, allowing for separation.
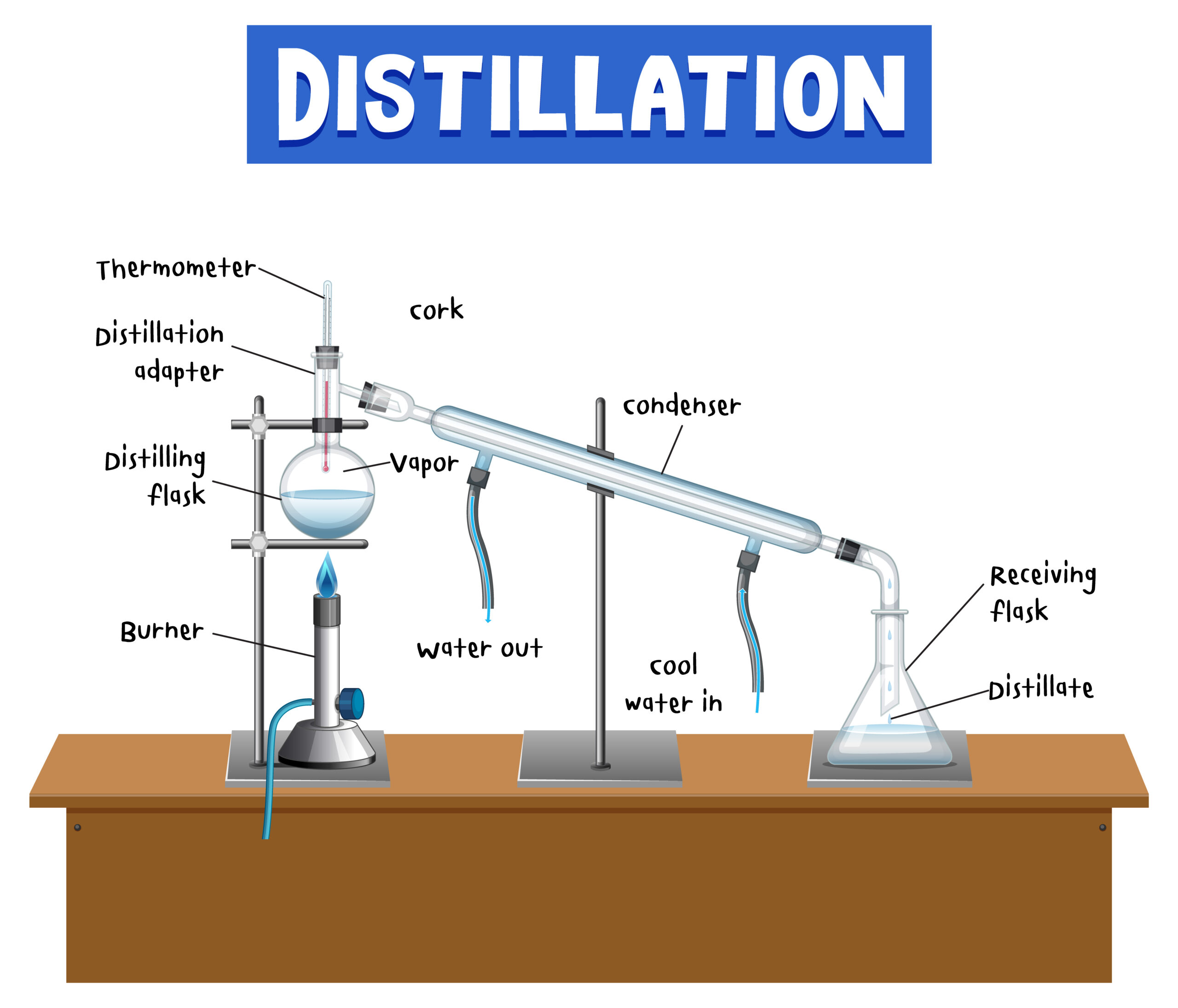
- Distillation: Differences in boiling points can be exploited for separation using distillation techniques.
Do check out other articles about organic chemistry in our category of Organic chemistry.

One comment
Comments are closed.