Myoglobin (Mb) and Haemoglobin (Hb) are both metalloproteins containing heme groups having distinctive red color with iron atoms, enabling them to bind and release oxygen. These two metalloproteins are present in Vertebrates & are large Polymeric entities. Hb transports oxygen throughout the body, while Mb stores and releases oxygen within muscle cells, optimizing oxygen availability for muscle function. Their distinct structures and functions make them essential players in the oxygen transport and utilization processes that are critical for sustaining life & physical activity.
Table of Contents
Myoglobin
The structural hallmark of Mb is its heme group, a Fe²⁺ chelated within a porphyrin ring. Mb is found in skeletal and cardiac muscle tissues. It is a monomeric protein, meaning it consists of a single polypeptide chain. Its primary function is to store and facilitate the release of oxygen within muscle cells. It has a higher affinity for oxygen than Hb, which allows it to capture oxygen from the bloodstream when oxygen levels are high (during inhalation) & then releases oxygen when oxygen levels within muscle cells decrease due to increased metabolic activity. This property helps to ensure that muscles have a readily available oxygen supply for energy production during strenuous physical activities.
Haemoglobin
Haemoglobin is an exquisitely crafted and indispensable protein that resides predominantly within the confines of erythrocytes, the red blood cells. It is a tetrameric protein, composed of four subunits, each containing a heme group with an iron atom at its core. Each subunit can bind to one molecule of oxygen. The tetrameric structure allows it to efficiently load and transport large quantities of oxygen from the lungs to various tissues and organs in the body where it is needed. Haemoglobin also plays a crucial role in transporting carbon dioxide from tissues back to the lungs for exhalation.
Active Site of Mb & Hb
The active site of myoglobin is located within a pocket in the protein’s structure, created by the folded polypeptide chain. At the center of this pocket is the heme group, which consists of a porphyrin ring with Fe²⁺ at its core. This Fe²⁺ serves as the primary binding site for oxygen. The iron can form coordinate bonds with the oxygen molecule. It’s active site has a hydrophobic environment, which enhances its oxygen-binding capacity. The proximity of the heme group to the protein surface facilitate rapid oxygen diffusion in and out of the active site.
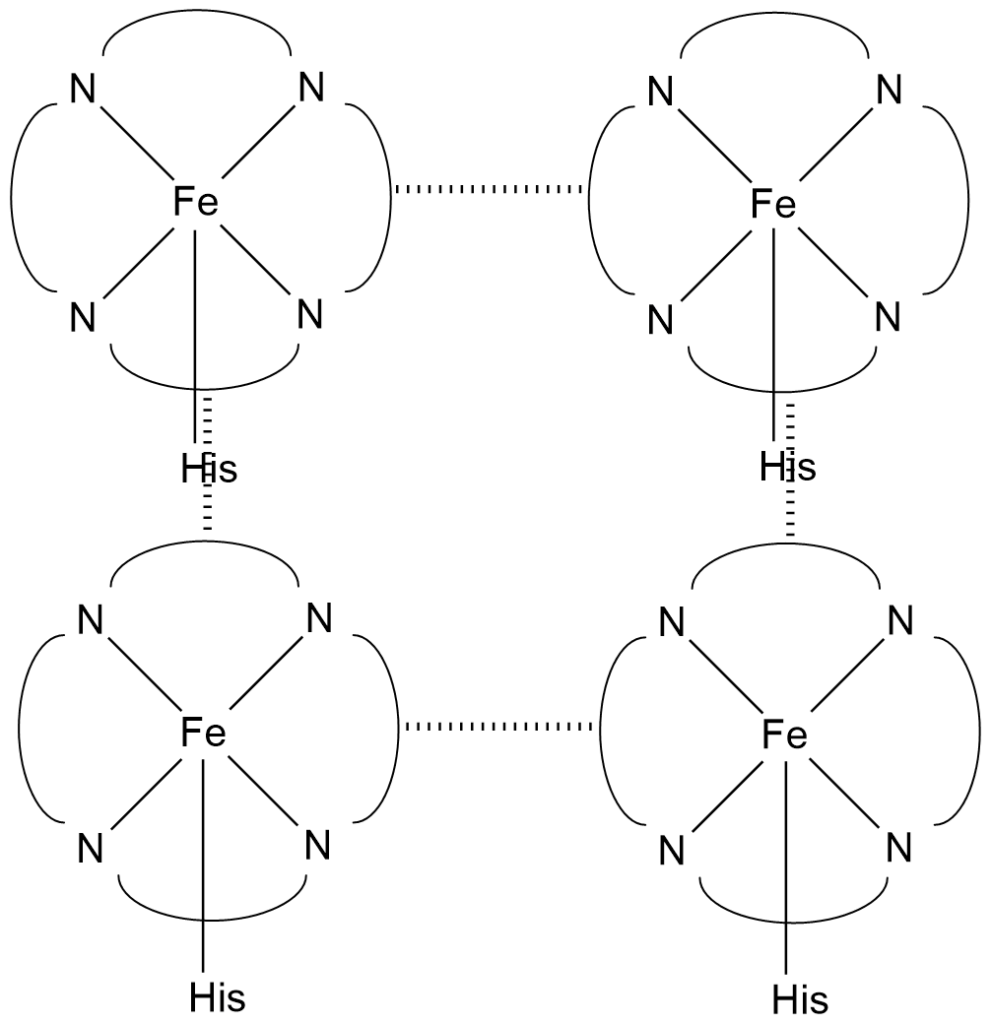
The active site of haemoglobin is similar to myoglobin but is more complex having multiple binding sites due to its tetrameric structure, comprising four globin subunits, each containing a heme group. Haemoglobin’s cooperative binding behavior is a key feature of its active site.
Deoxy & Oxy form of Mb & Hb
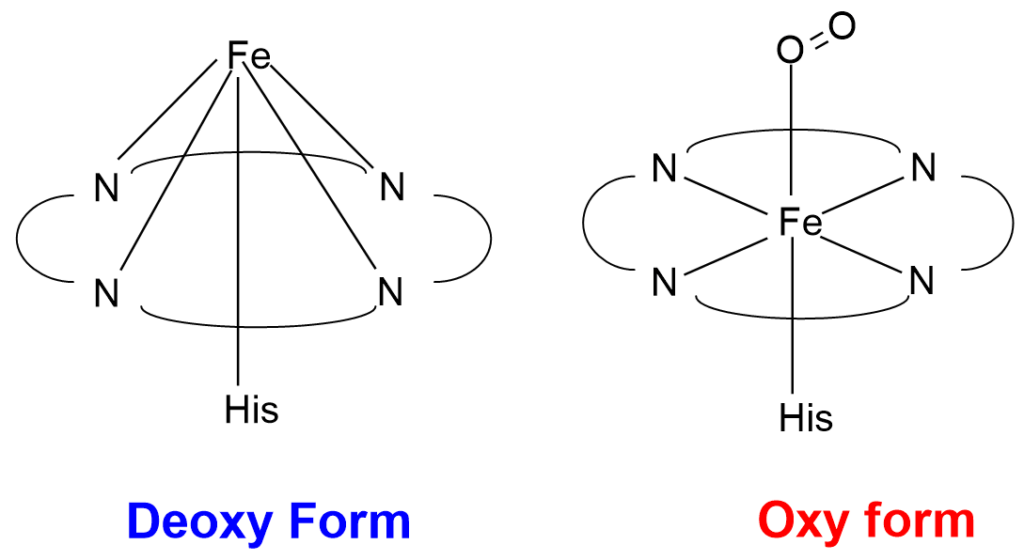
In Deoxy form, the Fe(II) is not present in the plane of the Porphyrin ring but is about 0.4A0 above the plane. Whereas in Oxy form Fe atom sits in the plane of the 4 porphyrin nitrogens. Deoxy-myoglobin refers to lack of oxygen molecule bound to Fe. Iron atom remains in a reduced ferrous state (Fe²⁺). Deoxy-Mb primarily exists in muscle tissue when oxygen levels are low or during periods of increased metabolic activity. In Deoxy-haemoglobin one or more of its subunits lack bound oxygen molecules. It typically occurs in tissues with higher oxygen demand, where oxygen has been released to support metabolic processes.
In Oxy form, Oxygen binds to Heme unit in the superoxide form having stretching frequency of 1100cm-1. Oxy-myoglobin is the oxygen-bound form of Mb. When oxygen levels in muscle tissue are sufficient, Mb captures oxygen from the bloodstream, and the oxygen molecule binds to the iron atom in the heme group. This binding results in the formation of oxy-Mb, which has a bright red color. Oxy-haemoglobin refers to the oxygen-bound form of hemoglobin. It predominates in oxygen-rich environments, such as the lungs, where it binds to oxygen molecules with high affinity. Oxy-Hb has a bright red color.
Conclusion
Mb serves as an oxygen reservoir in muscle cells, while Hb transports oxygen throughout the bloodstream to support the metabolic needs of various tissues and organs. These proteins respond to changes in oxygen levels, helping to maintain oxygen homeostasis and support the energy needs of various tissues during different physiological conditions.

2 comments
Comments are closed.