The projection formula for chiral molecules are used in organic chemistry to represent three-dimensional molecular structures in a two-dimensional format. Configuration of a chiral molecule is a three-dimensional structure and it is not easy to depict it on a paper having only two-dimensions. To overcome this problem the following two dimensional structures known as Projections have been evolved. There are several types of projection formulas, each serving a specific purpose in conveying molecular information. Here are some common types of projection formulae: Wedge dash notation, Fischer Projection, Sawhorse Projection, Newman Projection And Haworth Projection.
Table of Contents for Projection Formula
Wedge Dash Notation
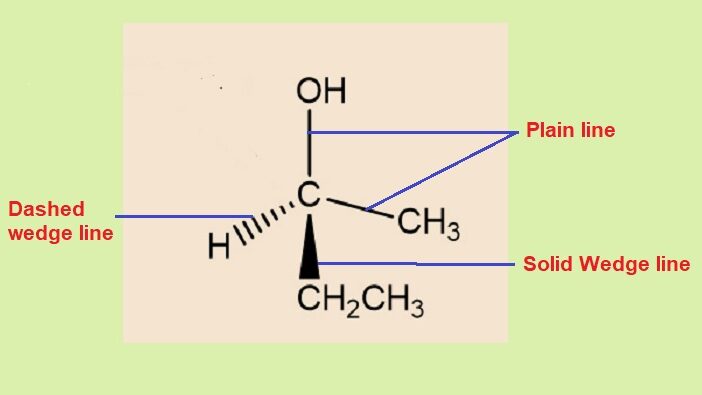
While not a traditional projection formula, wedge dash notation is often used to indicate the three-dimensional orientation of bonds in a flat structure. It is a method of representing the spatial arrangement of atoms in a molecule on a two-dimensional surface. •It is written in the plane of paper & is also called as “wedge dot formula”.
A solid wedge (upward pointing) represents a bond coming out of the plane of the paper toward the viewer, and a dashed wedge (downward pointing) represents a bond going into the plane of the paper away from the viewer. A plain line represents a bond in the plane of the paper.
The wedge dash notation of 2 enantiomeric lactic acid are:
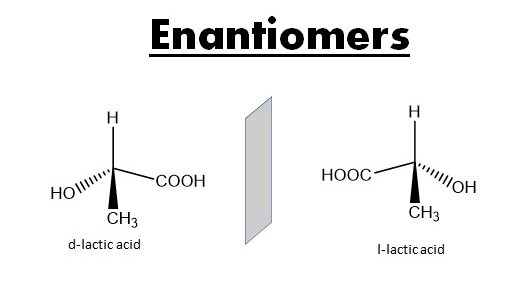
This notation is convenient for representing the molecules with 1 asymmetric Carbon atom & can be applied to a molecules into a symmetric Carbon atom but if there are more than 2 asymmetric Carbon atom than such a notation becomes complicated & is not suitable. Therefore in such case Fischer projection is used.
Fischer Projection
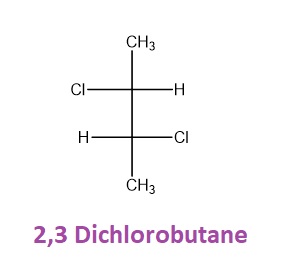
Emil fischer in 1891 introduced this method. Fischer projections are commonly used to represent stereochemistry, especially for chiral molecules. These projection are 2-D representation of 3-D molecule. In this projection, horizontal lines represent bonds coming out of the plane of the paper toward the viewer, and vertical lines represent bonds going into the plane of the paper away from the viewer. The horizontal lines are often interpreted as wedges, and the vertical lines as dashes, following the convention of wedge-dash notation. Here’s an example of a this Projection that is 2,3 Dichlorobutane.
Following points are to be observed for this purpose:
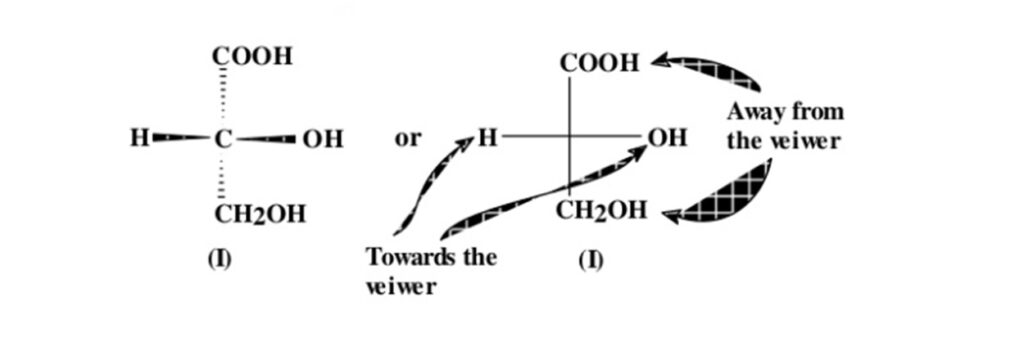
- Chiral molecule is imagined in a such a way that if the 2 groups point towards the observer & 2 groups away from the observer. The group pointing towards the observer are written along the horizontal line & those pointing away from the observer are written along the vertical line.
- The intersection of horizontal & vertical line represents are C atom usually the 1 that is stereogenic.
3.The longest chain of the C atom in a molecule should be represented along the vertical line.
4.The more oxidised group is placed at the top of the vertical line. The decreasing order of the oxidised state of some common group is as follows: -COOH > -CHO > -CH2-OH > -CH3
Characteristics Features of Fischer Projection:
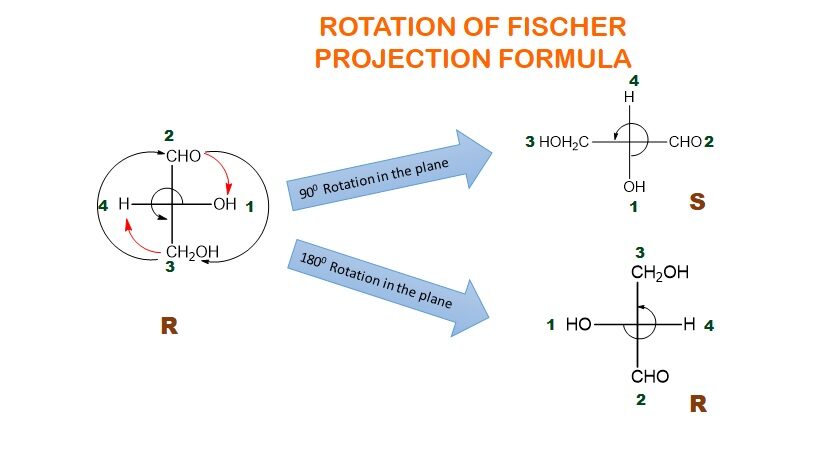
These Projections are particularly useful for representing stereocenters and chiral molecules. The spatial arrangement of substituents around a chiral center is easily conveyed. They are also used for non-chiral compounds, providing a simplified representation of molecular structures. Rotation of a Fischer Projection by an angle of 1800 about the axis which is perpendicular to the plane of the paper gives identical structures. However, similar rotation by an angle of 900 produces non-identical structure.
Sawhorse Projection
Sawhorse Projection are a hybrid between Fischer and Newman projections. It is particularly useful for visualizing the dihedral angle (torsional angle) between two substituents on a carbon-carbon bond. They show the carbon-carbon bond at an angle, providing a perspective between the front and side views. Here’s how to draw a Sawhorse projection:
- Draw the skeletal structure: Begin by drawing the skeletal structure of the molecule, focusing on the carbon-carbon bond of interest.
- Position the substituents: Place the substituents attached to each carbon on either side of the carbon-carbon bond, showing their relative positions.
- Indicate the bond angle: Draw a line connecting the substituents on each carbon to represent the carbon-carbon bond. This line is typically drawn at an angle, indicating the approximate tetrahedral bond angle.
- Label substituents: Label the substituents on each carbon to identify the specific groups or atoms attached.
The lower left hand carbon is taken as the front carbon or towards the observer and the upper right hand carbon as the back carbon or away from the observer.
Here’s a simple example of a Sawhorse projection for ethane:
In this example, the substituents on each carbon are represented, and the longer diagonal line connecting them indicates the carbon-carbon bond. The Sawhorse projection provides a visual representation of the dihedral angle between the substituents. Sawhorse projections are particularly useful for visualizing the relative orientations of groups around a carbon-carbon bond and understanding the conformational flexibility of molecules. sawhorse projections are much like wedge dash notation.
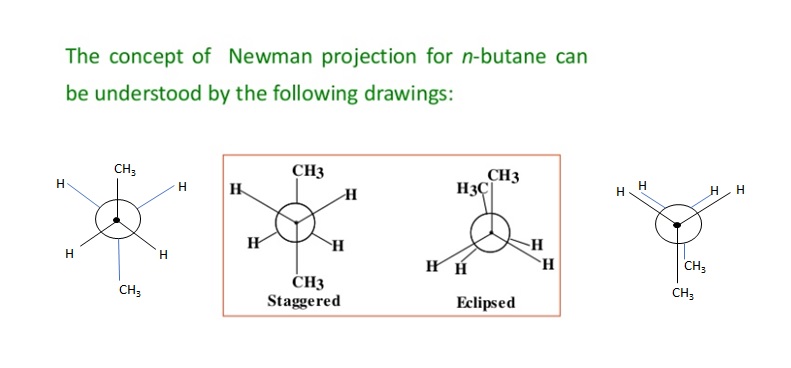
Due to free rotation along the central bond two extreme conformations are possible – the staggered and the eclipsed.
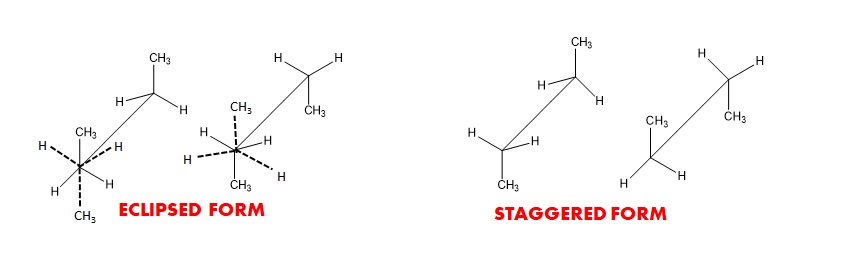
Newman Projection
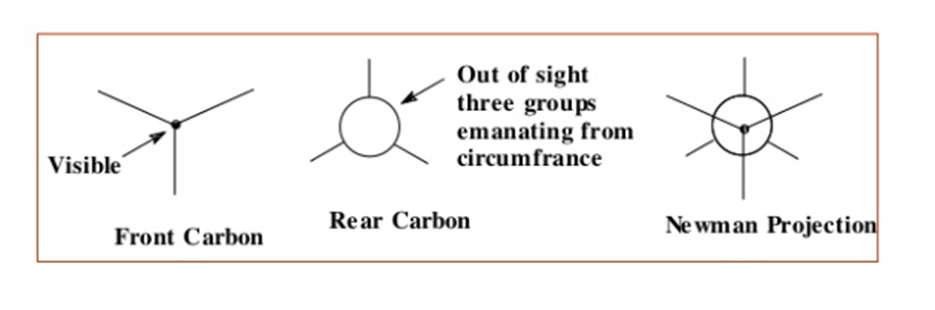
Newman devised a very simple method of projecting three dimensional formulas on two dimensional paper which are known as Newman projection. These projections are used to visualize the conformational structures of molecules and understanding the energy changes associated with bond rotations, especially those with single bonds that can rotate. In a this projection, you look down the bond axis, and the front carbon is represented as a circle, while the back carbon is a dot. Substituents are shown as lines projecting from the front carbon.
Here’s how to draw a Newman projection:
- Draw the skeletal structure: Begin by drawing the skeletal structure of the molecule, emphasizing the bond of interest.
- Choose a viewpoint: Select a viewpoint along the axis of the bond you want to represent. The front carbon is represented as a circle, and the back carbon is represented as a dot.
- Position substituents: Draw lines or curves connecting the substituents on each carbon to the center of the circle (front carbon) or dot (back carbon). The angle between the lines represents the dihedral angle or torsional angle.
- Label substituents: Label the substituents to identify the specific groups or atoms attached to each carbon.
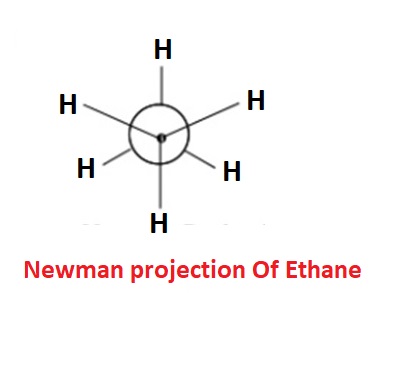
Here’s a simple example of a Newman projection for ethane in the staggered conformation:
This projection can be drawn only for the molecule having 2 or more C atom. It can be obtained by projecting the molecule such that the C-C bond perpendicular to the plane of paper. The molecule is viewed from the front or along the axis of a carbon-carbon bond. Hence rear C atom cannot be seen as it is covered by the front C atom. The carbon nearer to the eye is represented by a point and the carbon atom towards the rear by circle. The substituents on the carbon atoms are shown as being bonded to dot or circle by an angle of 1200 to each other.
In Newman Projection, all parallel bonds are eclipsed or all anti parallel or opposite bonds are staggered.
Haworth Projection
Haworth projections are commonly used for representing the cyclic structures of carbohydrates. They provide a top-down view of the ring structure, which is particularly useful for visualizing and understanding the arrangement of atoms within the cyclic molecule. This projection is named after the British chemist Sir Norman Haworth, who contributed significantly to the study of carbohydrates and their structures.
In a Haworth projection:
- The ring is typically drawn as a polygon, with the carbon atoms at the corners.
- The substituents on each carbon are usually shown as horizontal lines extending from the ring. For example, hydroxyl groups (-OH) are often represented as lines coming out of the right side of the ring.
- The orientation of these substituents indicates their stereochemistry, especially in the context of carbohydrates where chirality is common.
For example, a Haworth projection of Glucofuranose:
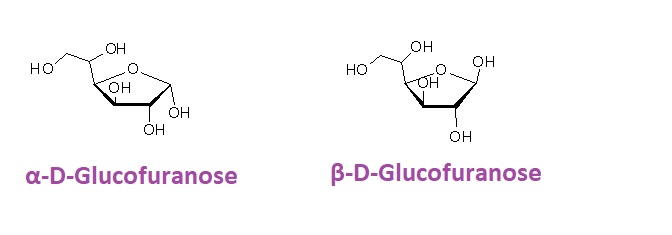
In this projection, the ring structure of glucofuranose is represented in a simplified and visually informative manner. The projection is especially valuable when dealing with larger and more complex carbohydrates, as it allows for a clear and concise representation of the cyclic structure.
Here are the key features and guidelines for drawing Haworth projections:
- Representation of Carbon Atoms: The carbon atoms in the ring are usually represented by corners of a polygon. For example, a sugar like glucofuranose would be represented as a five-membered ring.
- Placement of Functional Groups: The substituents or functional groups attached to each carbon are shown as either horizontal or vertical lines. Conventionally, horizontal lines represent substituents coming out of the plane of the paper toward the viewer, while vertical lines represent substituents going into the plane away from the viewer.
- Positioning of Heteroatoms: Heteroatoms (e.g., oxygen) are typically placed within the ring structure and are shown as part of the appropriate carbon’s horizontal or vertical line.
- Direction of Lines: The direction of the lines is used to convey stereochemistry. For example, in carbohydrates, the orientation of hydroxyl groups (OH) is important for indicating the specific isomer.
- Orientation of the Projection: The Haworth projection is a top-down view, so the atoms and groups are viewed from above the plane of the ring.
Projection formulae are used in organic chemistry for several reasons:
- Representation of Three-Dimensional Structures: Molecules exist in three-dimensional space, but it can be challenging to convey their structures accurately on a flat surface. Projection formulae provide a simplified, two-dimensional representation while preserving critical spatial relationships.
- Stereochemistry: Projection formulae, such as Fischer projections, are particularly useful for representing stereochemistry. They allow chemists to clearly depict the spatial arrangement of atoms around chiral centers and convey information about enantiomers and diastereomers.
- Conformational Analysis: Newman projections are valuable for studying the conformational flexibility of molecules, especially those with single bonds that can rotate freely. They provide insights into different conformations and help analyze the energy barriers associated with bond rotation.
- Ease of Communication: Projection formulae offer a concise and standardized way for chemists to communicate complex molecular structures. This is especially important in research, education, and communication within the scientific community.
- Simplification of Complex Structures: In cases where molecular structures are intricate, projection formulae simplify the representation, making it more manageable and easier to interpret. This is crucial when dealing with large or complicated molecules.
- Visualization of Ring Structures: Haworth projections are commonly used for cyclic compounds, especially in carbohydrates. They provide a top-down view of the ring structure, making it easier to visualize and understand the arrangement of atoms within the cyclic molecule.
- Concise Depiction of Conformations: Sawhorse projections, being a hybrid of Fischer and Newman projections, provide a middle ground between these two representations. They are useful for showing bond angles and conformations in a way that is more detailed than Fischer projections but less complex than Newman projections.
In summary, formulae serve as powerful tools for chemists to represent, analyse, and communicate the structures and properties of organic molecules. They are crucial in understanding the spatial arrangement of atoms, stereochemistry, and conformational flexibility.
Do check out other articles about organic chemistry in our category of Organic chemistry.