Aromaticity plays a crucial role in the chemistry of many natural and synthetic organic compounds, and it is an important concept for understanding chemical reactivity, the special stability and unique electronic structure of certain cyclic, planar, and conjugated compounds known as aromatic compounds. They can exhibit a higher degree of stability and reactivity compared to non-aromatic compounds with similar structures.
They are known for their unique chemical properties, including increased stability, resistance to addition reactions, and a tendency to undergo electrophilic aromatic substitution reactions rather than addition reactions.
Table of Contents
Aromatic examples
Benzene, Pyrrole, thiophene are some examples.
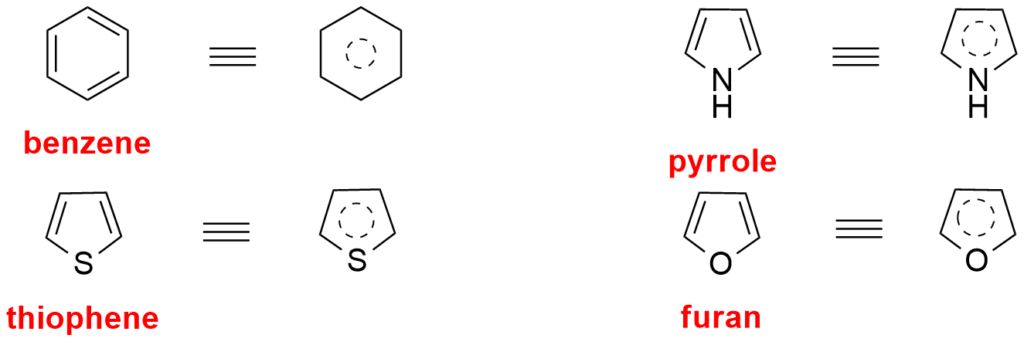
Key features of Aromaticity include
1. Cyclic Structure
Aromatic compounds are typically composed of a ring of carbon atoms, although heteroatoms (such as nitrogen, oxygen, or sulfur) can be included in the ring. The ring must be fully conjugated, meaning that the π electrons are delocalized throughout the entire ring.
2. Planarity
The atoms in the aromatic ring must lie in the same plane, allowing for effective overlap of π electrons. This planar arrangement contributes to the stability of the compound.
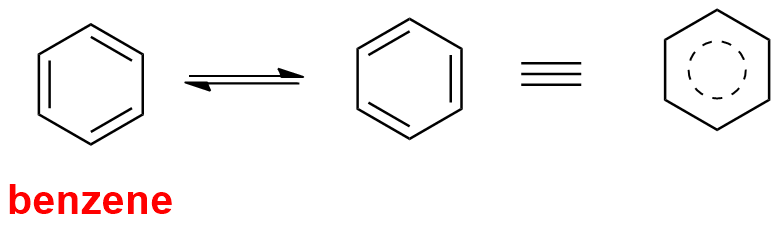
3. Presence of high reasonance energy
Stability of a compound is explained by reasonance. The greater is the number of reasonating structures, the more stable is the molecule or compound. The resonating or Kekule structures of benzene are as:
4. Conjugation
Aromatic compounds have a continuous system of alternating single and double bonds (π electrons) around the ring. This conjugated π electron system results in enhanced stability.
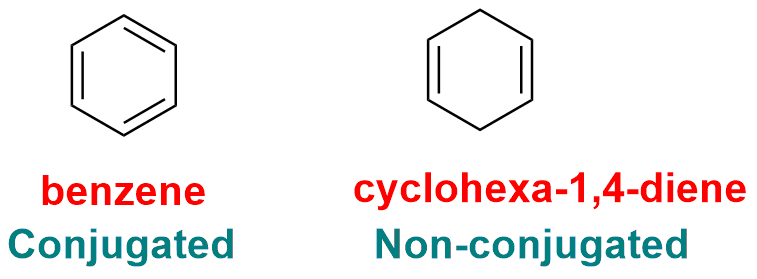
5. Presence of Delocalization of π-electron
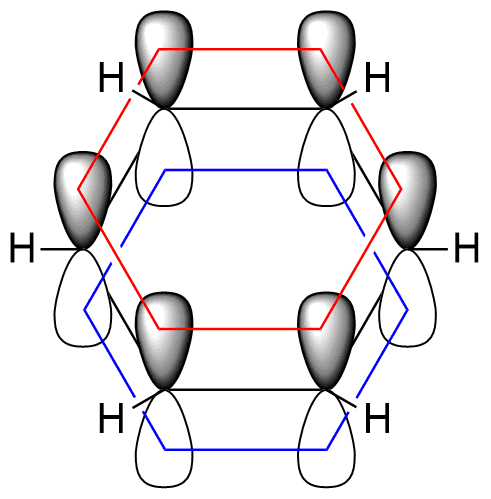
Benzene, a hexagonal molecule, features six carbon and six hydrogen atoms in a flat plane with a 120-degree valency angle. Through sp2 hybridization, each carbon retains an unhybridized 2p-orbital. These six 2p-orbitals are evenly spaced and parallel, overlapping to create a delocalized molecular orbital encircling all six carbon atoms in the benzene ring. This delocalization of π-electrons results in the energy minimization of the benzene molecule, enhancing its stability.
6. Hückel’s Rule
Hückel’s Rule is a mathematical criterion used to determine whether a planar, cyclic compound is aromatic. According to this rule, a compound is aromatic if it contains 4n + 2 π electrons, where “n” is an integer (0, 1, 2, 3, etc.). Compounds that satisfy this rule are considered aromatic, while those that do not are non-aromatic.
Classification Of Aromatic Compounds
This can be classified into two main categories:
1. Benzenoid
These compounds have a benzene ring as their core structure. Benzene itself is the most well-known example of a benzenoid aromatic compound. Other examples include toluene, phenol, and aniline.
2. Non-Benzenoid
These are the compounds that do not have a benzene ring but still satisfy the criteria for aromaticity. Examples include pyridine, furan, and pyrrole. Aromaticity of Non-Benzenoid Aromatic Compounds can be known by Hückel’s Rule & Craig’s Rule. Hückel’s Rule is used in monocyclic polyenes while Craig’s Rule is used in bicyclic and polycyclic polyenes.
Polycyclic Aromatic compounds
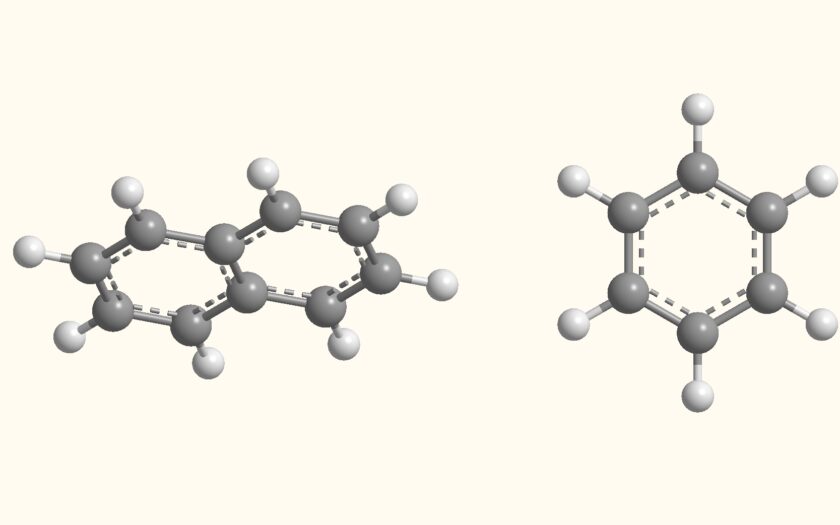
Polycyclic aromatic compounds are a class of organic compounds characterized by the presence of multiple aromatic rings that are fused together in a cyclic structure. These compounds are composed of two or more aromatic rings, also known as benzene rings, which share common carbon atoms along their edges. They can vary in size and complexity, with some containing just a few fused rings, while others are highly complex with numerous rings.
One of the most well-known polycyclic aromatic compounds is naphthalene, which consists of two fused benzene rings. Another common example is anthracene, which has three fused benzene rings. Even more complex polycyclic aromatic compounds include pyrene and benzo[a]pyrene, which contain multiple fused rings. Get more article on Organic chemistry on our organic category.