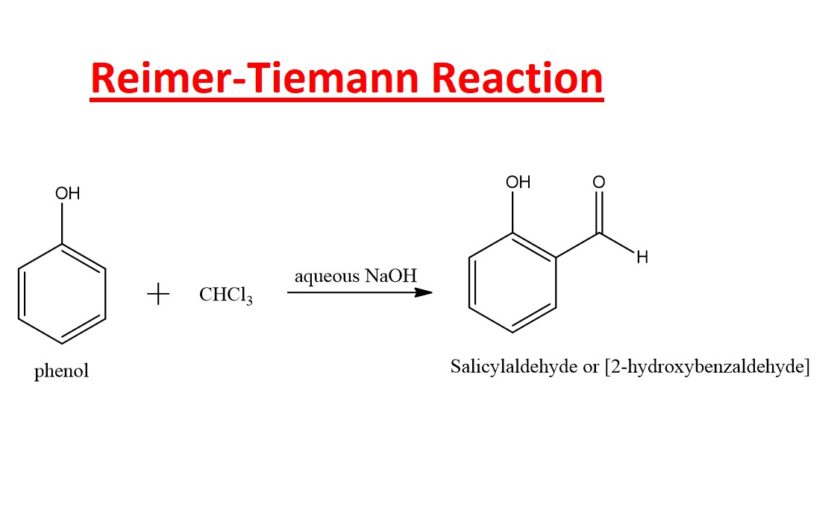
The Reimer Tiemann reaction is a fundamental organic transformation that involves the conversion of phenols (aromatic compound containing a hydroxyl group) into salicylaldehyde (2-hydroxybenzaldehyde) by using chloroform and a strong base in the presence of water and heat. This reaction is of considerable importance in both synthetic chemistry and the pharmaceutical industry due to its role in the synthesis of salicylaldehyde, a key intermediate in the preparation of various salicylate-based drugs such as aspirin. In this comprehensive article, we will delve into the Reimer Tiemann reaction, exploring its mechanism, applications, variations, and historical significance.
Table of Contents
Introduction
Overview of the Reimer Tiemann Reaction
The Reimer Tiemann reaction is classified as a formylation reaction, which means it introduces a formyl group (-CHO) at the ortho position with respect to the hydroxyl group onto an aromatic ring. This reaction is particularly useful in the synthesis of salicylaldehydes, which are key intermediates in the production of aspirin, a widely used analgesic. Understanding the mechanism, scope, and applications of the Reimer Tiemann reaction is crucial for chemists involved in medicinal chemistry, drug development, and related fields.
Historical Context
The Reimer Tiemann reaction was first reported independently by Karl Reimer in 1876 and Ferdinand Tiemann in 1880. This reaction was developed during a time when the understanding of organic chemistry was still evolving, and the detailed mechanistic insights that we have today were not available. Despite the lack of mechanistic knowledge at the time, chemists were able to exploit this reaction for the synthesis of salicylaldehydes, which had various applications in the dye and pharmaceutical industries.
Mechanism of the Reimer Tiemann Reaction
The Reimer Tiemann reaction proceeds through several steps, which involve the formation of a reactive intermediate called a carbene. It is important to note that the carbene intermediate plays a pivotal role in this reaction, and its formation and subsequent reactivity are central to the success of the Reimer Tiemann reaction. The general mechanism is as follows:
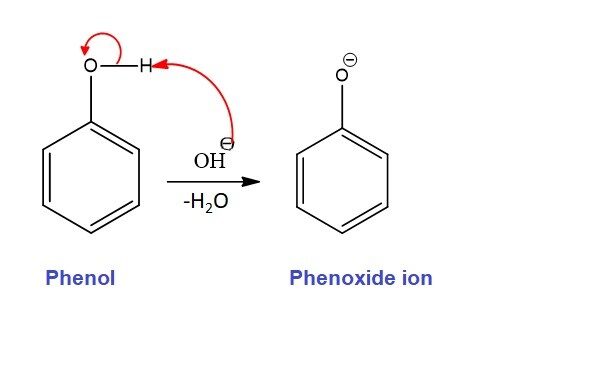
Step 1: Formation of Dichlorocarbene & Phenoxide ion
The reaction typically starts with the mixing of a phenol (ArOH), chloroform (CHCl3). The deprotonation of chloroform (CHCl₃) by a strong base, typically sodium or potassium hydroxide (NaOH or KOH). This generates a dichlorocarbene intermediate, which is a highly reactive electrophile species and plays a crucial role in the subsequent steps of the reaction.
CHCl₃ + NaOH → CCl₂ + NaCl + H₂O
The strong base also deprotonates the phenol to form the phenoxide ion (ArO⁻), where the oxygen of the phenol has lost a proton, becoming negatively charged. The negative charge on oxygen is stabilized by resonance with the aromatic ring..
Step 2: Addition of Dichlorocarbene & Rearrangement
The dichlorocarbene attacks the phenol’s aromatic ring through electrophilic aromatic substitution. The dichlorocarbene is attracted to the electron-rich aromatic ring. This step results in the formation of an intermediate compound which undergoes a rearrangement where there is loss of Chlorine & formation of 6-(chloromethylene)cyclohexa-2,4-dienone.
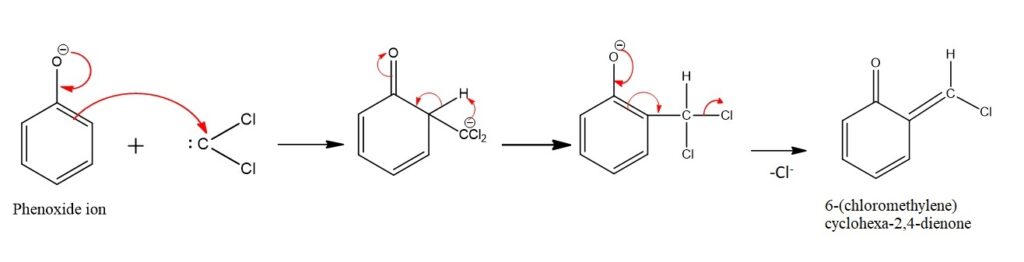
Dichlorocarbene can add to either of the two ortho positions with respect to the hydroxyl group. The reaction’s regioselectivity often favors the ortho position, but some meta products can also form, depending on the specific conditions.
Step 3: Hydrolysis
6-(chloromethylene)cyclohexa-2,4-dienone is then hydrolyzed by the base (NaOH) and heat, leading to the cleavage of the carbon-chlorine bonds. This results in the formation of salicylaldehyde and sodium chloride (NaCl). This step leads to the replacement of the chloroform moiety with a hydroxyl group (−OH) and the generation of a carbonyl group (−C=O). The hydrolysis step completes the transformation, and salicylaldehyde is obtained as the desired product.
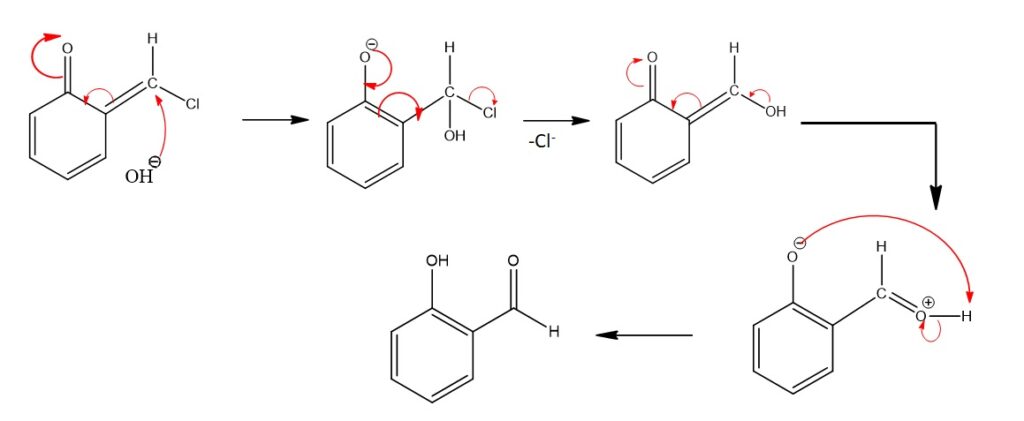
Step 4: Tautomerization
Salicylaldehyde is in equilibrium with its tautomeric form, the enol. Tautomeric forms are constitutional isomers that can interconvert rapidly by the migration of a proton. In the case of salicylaldehyde, the enol form is stabilized by hydrogen bonding:
aldehyde form ⇌ Enol form
The aldehyde form is the one typically isolated and used in further reactions.
Reagents and Conditions
Chloroform (CHCl3)
Chloroform is a key reagent in the Reimer Tiemann reaction. Chloroform is the carbene precursor in this reaction. It provides the dichlorocarbene needed for the reaction to proceed. The chloroform should be dry, as the presence of water can lead to side reactions.
Phenol
The starting material for the reaction is typically a phenolic compound. It can be a simple phenol or a more complex compound containing a phenolic group.
Base (usually aqueous sodium hydroxide, NaOH)
A strong base is essential for deprotonating the phenol to form the phenoxide ion (ArO⁻) and for initiating the reaction. It deprotonates chloroform to generate the dichlorocarbene ion. Sodium hydroxide is commonly used due to its availability and strong basicity.
Solvent and Temperature
The reaction is often carried out in a suitable solvent, such as ethanol or methanol, to facilitate mixing and heat dissipation and is typically performed at or slightly above room temperature.
Applications
The Reimer Tiemann reaction has found diverse applications in organic synthesis and plays a crucial role in the preparation of numerous chemical compounds. Some notable applications include:
- Salicylaldehyde Synthesis: The primary application is the conversion of phenolic compounds into salicylaldehydes, which are crucial intermediates in the synthesis of various salicylate-based drugs, fragrances, and other chemicals. For instance, salicylic acid, an aspirin precursor, is derived from salicylaldehyde.

- Pharmaceuticals: Salicylates, including aspirin, are widely used over-the-counter drugs for their anti-inflammatory, analgesic, and antipyretic properties. The Reimer Tiemann reaction plays a vital role in the synthesis of salicylate-based drugs and derivatives.
- Agrochemicals: Salicylic acid and its derivatives are also used in agrochemicals. They serve as essential components of plant growth regulators and are involved in the induction of systemic acquired resistance (SAR) in plants, which enhances their resistance to pathogens.
- Flavor and Fragrance Industry: Salicylaldehyde is a key ingredient in the production of fragrances and perfumes. Its pleasant, sweet, and floral odor makes it a valuable component in the fragrance industry.
- Dyes and Colorants: The Reimer Tiemann reaction is employed in the preparation of dyes and colorants for textiles and other applications.
- Aromatic Chemistry: It serves as a valuable tool in the synthesis of aromatic compounds, allowing for the introduction of formyl groups at specific positions on the aromatic ring.
- Research and Education: The reaction is commonly used in educational settings to demonstrate key concepts in organic chemistry.
Scope and Limitations
The Reimer Tiemann reaction is versatile and applicable to a wide range of phenols and related compounds. However, there are certain limitations and considerations:
- Substrate Requirements: The phenol substrate should be electron-rich and have an ortho- or para-directing group (e.g., -OH, -OCH3, -NH2) for efficient formylation. It has limited applicability to other substrates.
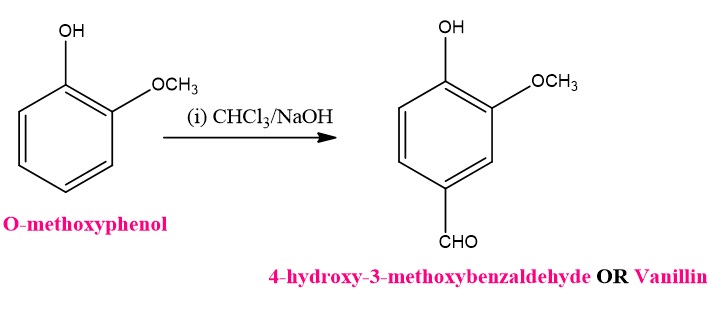
- Steric Hindrance: Bulky substituents on the aromatic ring may hinder the reaction due to steric effects.
- Reactivity of Phenol Derivatives: The success of the reaction is highly dependent on the nature of the phenol substrate. Phenols with electron-donating groups (e.g., methoxy groups) on the aromatic ring are generally more reactive and yield better results. Phenols with strong electron-withdrawing groups are less reactive in the Reimer Tiemann reaction.
- Side Reactions: Depending on the reaction conditions, side reactions like halogenation of the aromatic ring or over-formylation may occur.
- Toxicity: Chloroform, a key reagent, is toxic and poses environmental and health risks. Green chemistry approaches aim to address this issue.
- Waste Generation: The reaction generates byproducts, including sodium chloride (NaCl), which may require additional disposal considerations.
Variations and Modifications:
While the classical Reimer Tiemann reaction involves the use of chloroform as the carbene precursor, there are variations and modifications of the reaction that employ different reagents and conditions. Some of these include:
- Reimer-Tiemann Carboxylation: This variation uses carbon tetrachloride (CCl4) and sodium hydroxide to introduce a carboxyl group (−COOH) instead of a formyl group.
- Tiemann Reaction: The Tiemann reaction is a modification that employs bromine (Br2) and sodium hydroxide to convert phenols into brominated aromatic compounds.
- Reimer Tiemann Reaction with Other Bases: While sodium hydroxide is commonly used, other strong bases like potassium hydroxide (KOH) or sodium ethoxide (NaOC2H5) can be employed in the reaction. The choice of base can influence the reaction rate and product selectivity.
- Green Chemistry Approaches: Researchers have explored greener alternatives to the Reimer Tiemann reaction, aiming to minimize the environmental impact by using alternative solvents and reagents.
- Using Other Halogens: Although the traditional Reimer Tiemann reaction involves chloroform (CHCl3), similar reactions can be carried out using other halogenated compounds such as bromoform (CHBr3) and iodomethane (CH3I), have been used to generate different carbenes. These variations can lead to the synthesis of structurally diverse compounds.
- Solvent Choices: Traditionally, water is used as the solvent in the Reimer Tiemann reaction, but variations using other solvents, such as alcohols, have been explored. These solvent modifications can influence reaction outcomes and efficiency.
- Phase-Transfer Catalysis: The use of phase-transfer catalysts in the Reimer Tiemann reaction allows the reaction to proceed more efficiently when the starting materials are not easily soluble in the reaction medium. Phase-transfer catalysts facilitate the movement of reagents between different phases, promoting reaction progress.
- Microwave-Assisted Reimer-Tiemann Reaction: Microwave irradiation has been employed to accelerate the Reimer Tiemann reaction, reducing reaction times and improving yields. The application of microwave energy can enhance the heating and mixing of reaction components, leading to more efficient reactions.
These variations and modifications demonstrate the adaptability of the Reimer Tiemann reaction to different reaction conditions and goals, making it a versatile tool in synthetic organic chemistry.
Conclusion
The Reimer Tiemann reaction represents a powerful tool in the arsenal of synthetic organic chemists. Its simplicity and efficiency in converting phenols into salicylaldehydes have made it a widely used reaction in both academic and industrial settings. While the basic mechanism involves the formation and reactivity of a dichlorocarbene intermediate, variations and modifications of the reaction have expanded its scope and applicability.
Understanding the mechanism, reaction conditions, and applications of the Reimer Tiemann reaction is essential for chemists working in organic synthesis and related fields. By mastering this reaction, researchers can access a wide range of valuable compounds with diverse applications in science and industry.
For more article regarding organic chemistry checkout the category of organic.